 Artist’s impression of a red giant star. Their cores are cauldrons where carbon-12 is produced. Credit:NASA/ Walt Feimer
Artist’s impression of a red giant star. Their cores are cauldrons where carbon-12 is produced. Credit:NASA/ Walt Feimer
Each of us is, as it says in Max Ehrmann’s famous poem “Desiderata”, a child of the universe. It speaks metaphorically about our place in the cosmos, but it turns out to be a very literal truth. Our bodies contain the stuff of stars and galaxies, and that makes us children of the cosmos. To be more precise, we are carbon-based life forms. All life on Earth is based on the element carbon-12. It turns out this stuff is a critical gateway to life. So, how did the universe come up with enough of it to make you and me and all the life on our planet? Astrophysicists and nuclear physicists think they have an answer by using a supercomputer simulation of what happens to create carbon. As it turns out, it’s not very easy.
The recipe for carbon-12 requires a pressure cooker and a lot of source material. The environment inside a star or during a stellar collision or an explosion provides the pressure cooker. The ingredients inside are helium-4 atoms and a theoretically forbidden nucleus of something called beryllium-8 (8Be). Put them all together and eventually, you get carbon-12. Sounds simple, right?
Well, not exactly. There’s no way to replicate this recipe in the lab to test it and prove the process. That’s because you need temperatures and pressures that exist only inside stars. To understand why we can’t reproduce the birth of carbon, here’s a simple outline of a complex process that astrophysicists think is happening.
Diving into Stars
The cores of stars engage in a process called nucleosynthesis. That’s a fancy term for “cooking up new elements.” So, for most of its life, a star is a giant sphere of hydrogen. Inside, the conditions are just right for it to fuse hydrogen atoms to make helium atoms. Take it a step further, and fuse two helium nuclei together, and you get that 8Be. There’s a problem though: the beryllium isotope should not exist. So, we’re at an impasse. If these things can’t be made, you don’t get carbon, and life maybe doesn’t get to exist. What’s the solution? Not silicon-based life forms (as we often hear about in science fiction), or, we still wouldn’t exist. (At least not in our current form.)
Getting to Carbon-12
So, carbon is important. It’s everywhere. Carbon is the fourth most abundant element after hydrogen, helium, and oxygen. The Big Bang created hydrogen and helium. Stars make oxygen and carbon. Older stars such as red giants are prodigious producers of carbon and they are everywhere. So, obviously, it happens. How? It turns out that things are fast and furious inside a star. The “forbidden” 8Be actually can exist, even if only for brief moments. However, it decays in a few nanoseconds.
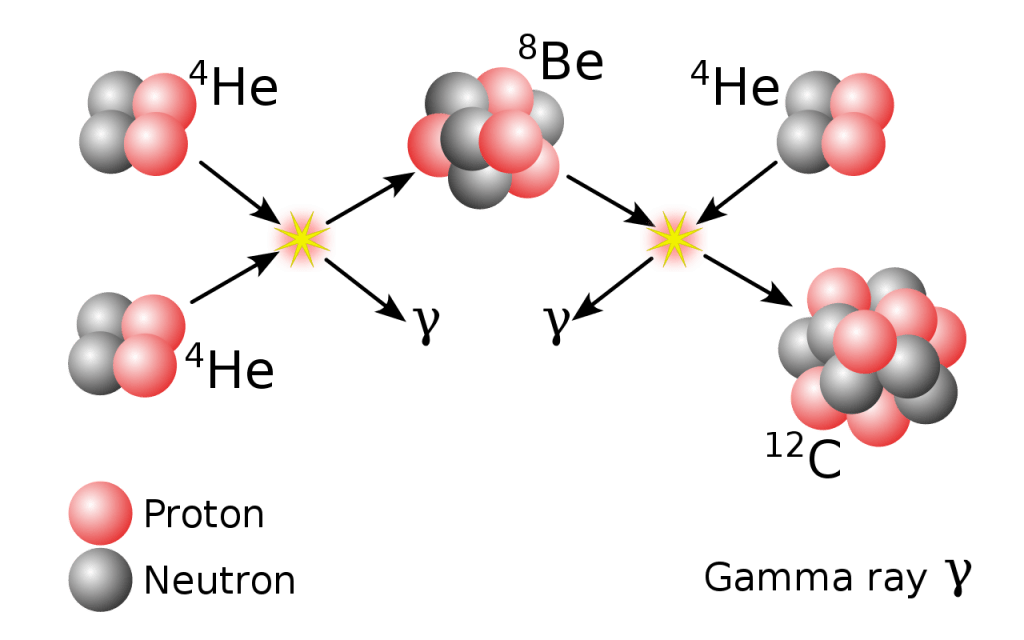 A graphical look at the triple-alpha process that produces carbon-12. Image credit: Borb, CC BY-SA 3.0.
A graphical look at the triple-alpha process that produces carbon-12. Image credit: Borb, CC BY-SA 3.0.
So, there isn’t much time to create carbon-12. But, it happens, or we wouldn’t be here to talk about it. Here’s how. There’s a chemical reaction involving three helium nuclei called “alpha particles” and the very short-lived 8Be. If conditions are just right inside the star, the alpha particles and the 8Be can fuse together. This forms the basis for carbon-12 production. This is the “triple alpha” process and occurs inside these older stars with extremely hot interiors (like above 108 K).
Modeling the Formation of Carbon-12
We can’t look inside an old red giant star to see what happens inside. Nor can we send a probe into a stellar collision. No spacecraft can go through a stellar explosion to witness elemental creation in those events. So, how do we know that this process is the correct one to explain the creation of carbon-12? As is often the case in astrophysics, researchers turn to computer models. Scientists at Iowa State University and the University of Tokyo created a complex supercomputer model of how alpha particles (the helium-4 atoms) and the 8Be nuclei combine to form much heavier atoms. In their model, this reaction created an unstable, excited carbon-12 state called a Hoyle state. (Named for astrophysicist Fred Hoyle who predicted this state in 1953.)
First, the researchers created a robust simulation of the environment and processes responsible for creating carbon-12 inside stars. Then, they ran it on the Fugaku supercomputer at the RIKEN Center for Computational Science in Kobe, Japan. The team also developed new techniques in computational artificial intelligence that would reveal alpha clustering in the Hoyle state implicated in the creation of carbon-12.
“There’s a lot of sublety—a lot of beautiful interactions going on in there,” said James Vary of Iowa State University. He is the first author of the resulting research paper. The research team points that alpha-particle clustering “is a very beautiful and fascinating idea and is indeed plausible because the (alpha) particle is particularly stable with a large binding energy.”
Ideas to Pursue in the Future
Now that the basic question of carbon-12 production seems to be answered by the simulations, Vary raises other questions about it. “Was carbon production mostly the result of internal processes in stars?” he asked. Or, could it also be in supernova explosions? Could it happen in the collisions of neutron stars? Recent studies show that such mergers create gold and platinum. So, were conditions ripe for carbon-12 to come about?
There are still many details to be worked out before researchers have a complete understanding of the carbon-12 process. But, understanding the role of nucleosynthesis is a prime goal. “his nucleosynthesis in extreme environments produces a lot of stuff,” Vary said, “including carbon.”
For More Information
Alpha-clustering in atomic nuclei from first principles with statistical learning and the Hoyle state character (research paper for this story), Nature Communications, April 27, 2022.
More about Nucleosynthesis from Universe Today podcast
On the Origin of Life’s Most Crucial Isotope (from Phys.org).
Researchers reveal the origin story for carbon-12, a building block for life

